Simple animal communities on Antarctic nunataks

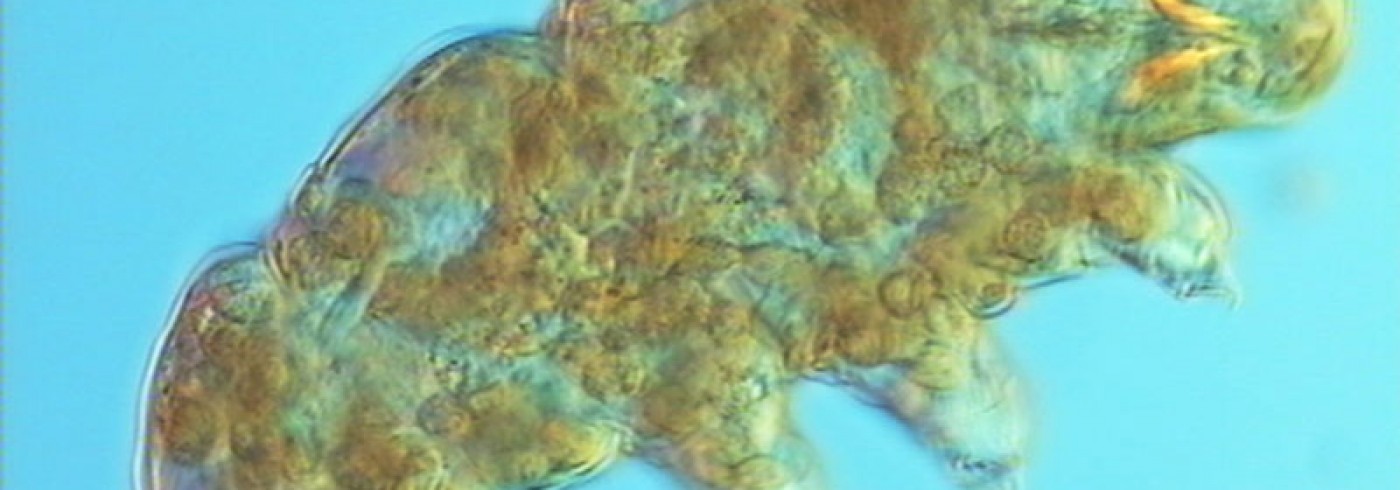
Specimens of the genus Plectus are the most common nematodes on the nunatak Basen in Dronning Maud Land. The SEM-picture shows the anterior end of a female. Photo: Sven Boström
On Antarctic nunataks (mountain peaks penetrating the ice shield) there exist simple communities of microscopic multi-cellular animals, which are dependent on water or water films for an active life. These animals, which all have the ability to survive drying and freezing, belong to the phyla Nematoda (roundworms), Rotatoria (wheel animalcules), and Tardigrada (water bears). The microfauna is organised into very simple and species-poor communities, which are of great interest for studies of basic ecological problems such as food web interactions, dispersal and meta-population ecology (Wall and Virginia, 1999).
The largest numbers of animals are generally found in places with visible plant production but animals are also found in places with no signs of macroscopic plant growth (Sohlenius et al., 1996). The basis of the food web consists of patches of mosses, lichens and blue-green bacteria (algae). Animals feeding on primary producers may be found in all three animal groups. The detritus pathway, including bacteria and fungi, is also of great importance for these animal communities. Among the nematodes found in Antarctica most species feed on bacteria or unicellular algae. There are no, or very few, fungal feeding nematode species. Some omnivorous nematode species feeding on soil algae or on other microscopic animals are occasionally found. Predators are also found among the tardigrades, e.g. the genus Macrobiotus. Other tardigrades may be plant or microbial feeders, e.g. the genera Hypsibius, Hebesuncus and Diphascon.
The communities of microfauna are patchily distributed and very species-poor. Only two bacterial feeding genera of nematodes (Plectus and Panagrolaimus) are found in high population densities. Members of these genera obviously prefer different kinds of habitats (Boström, 1995). Plectus is particularly abundant in moss cushions, whereas Panagrolaimus is particularly abundant in the vicinity of birds’ nests, often within mats of blue-green bacteria (Prasiola crispa). The patterns of distribution of the tardigrade species differ, but further analyses are necessary to show if these patterns can be related to different habitat requirements among the species.

Suspension with microscopic animals extracted from organic material obtained from an Antarctic nunatak. The suspension contains one nematode (Panagrolaimus), five tardigrades (Diphascon), and seven rotifers. Photo: Björn Sohlenius
The nunataks are often separated by long distances of ice and within each nunatak suitable habitats are sometimes located in rather isolated patches. This means that many animal populations are extremely isolated and that the probability of dispersal into new areas is certainly low. If the probability of reaching a suitable habitat is limited, it might be expected that there are suitable habitats which have not been colonised and thus are devoid of animals. The patterns of distribution of nematode and tardigrade species observed so far indicate that their dispersal is hindered by barriers, e.g. long stretches of ice-covered landscape (Sohlenius et al., 1996).
During the Swedish Antarctic expedition (SWEDARP) 2001/02, 214 samples of soil material, including algae, mosses and lichens, were collected by Ingemar Jönsson on nunataks in Vestfjella and Heimefrontfjella and from the area around the Russian station Novolazarevskaya in Dronning Maud Land. Most of the samples were extracted at the Swedish station Wasa by a wet-funnel method (Sohlenius, 1979), but some were dried and later extracted in Stockholm. The suspensions of animals were killed by heat and fixed in cold TAF (triethanolamine and formalin). For light microscopy they were transferred to anhydrous glycerine by a slow evaporation method. The abundance of animals and fauna composition was determined for each sample.
The results obtained 2001/02 will be integrated with fauna data from earlier Swedish Antarctic expeditions (Sohlenius et al., 1995, 1996). One aim of the studies is to get a picture of the community structure and of the patterns of spatial distribution of the animals. The composition and distribution of the microfauna can also be used to monitor environmental changes due to human activities.

Abundance and composition of the microfauna on Antarctic nunataks (Basen, Fossilryggen, Plogen and Steinnabben) and at the station Novolazarevskaya. Figures show mean abundance as numbers per gram material dry weight (Abund No/gdw) in samples where the animal group occurred, and frequency of occurrence as numbers (Freq No) and percent (%) of samples where the animals occurred. The table shows values for all samples, separate values for samples containing organic material or only mineral material, and for different kinds of vegetation e.g. mosses, lichens and algae.
Members of any of the three groups nematodes, rotifers and tardigrades were found in 87% (187) of the 214 analysed samples (table 1). The most widely dispersed phylum was the rotifers, which was found in 72% of the samples. Nematodes and tardigrades had a more restricted distribution and occur-red in 44% and 47% of the samples, respectively. The population densities in the patches of organic material were sometimes very high, with peak abundances of 2 500 nematodes, 4 200 rotifers and 120 tardigrades per gram of material (dry weight). There were large differences in fauna composition between the substrates mosses, lichens and algae. Thus the richest diversity was found in moss cushions with Plectus acuminatus as the dominating nematode species. In association with bird colonies there were extensive growths of the blue-green bacterium (alga) Prasiola crispa, which supported very high numbers (up to 2 500 specimens/gram dry weight) of the nematode species Panagrolaimus magnivulvatus. In these places there were no predatory tardigrades (Macrobiotus), which might depress the numbers of Panagrolaimus. In other places where Macrobiotus occurred abundantly, lower numbers of nematodes and rotifers were found. The mean numbers of nematodes and rotifers were 35 and 17 specimens/gram dry weight, respectively, in the presence of Macrobiotus, while there were 214 nematodes and 129 rotifers/gram dry weight where Macrobiotus was not found. This is an interesting indication of food web influences by predation. These relationships will be further analysed including the material from previous expeditions.
In the vicinity of the station Wasa occasional occurrences of nematode species not native to Antarctica may indicate contamination due to human activities.
Dates
November 2001–February 2002
Participants
Principal investigator
Björn Sohlenius*
Department of Invertebrate Zoology, Swedish Museum of Natural History
Stockholm, Sweden
Sven Boström*
Department of Invertebrate Zoology, Swedish Museum of Natural History
Stockholm, Sweden
K. Ingemar Jönsson
Department of Theoretical Ecology, Lund University
Swedem
*Not participating in the expedition
References
Boström, S. 1995. Populations of Plectus acuminatus Bastian, 1865 and Panagrolaimus magnivulvatus n. sp. (Nematoda) from nunataks in Dronning Maud Land, East Antarctica. Fundamental and Applied Nematology 18, 25–34.
Sohlenius, B. 1979. A carbon budget for nematodes, rotifers and tardigrades in a Swedish coniferous forest soil. Holarctic Ecology 2, 30–40.
Sohlenius, B., Boström, S. and Hirsch-felder, A. 1995. Nematodes, rotifers and tardigrades from nunataks in Dronning Maud Land, East Antarctica. Polar Biology 15, 51–56.
Sohlenius, B., Boström, S. and Hirsch-felder, A. 1996. Distribution patterns of microfauna (nematodes, rotifers and tardigrades) on nunataks in Dronning Maud Land, East Antarctica. Polar Biology 16, 191–200.
Wall, D.H. and Virginia, R.A. 1999. Controls on soil biodiversity: insights from extreme environments. Applied Soil Ecology 13, 137–150.